After several years of waiting and several amendments, the EU published the final revision document of Annex 1 to Good Manufacturing Practices (GMP).
As stated in the opening of the document itself, the purpose of revising Annex 1 is to reflect the changes that have taken place in the legislation and production of sterile pharmaceutical products since the first version in 2007. Actually, the novelties contained in the 10 sections that make up the new version of Annex 1 are many. Starting with the chapter on isolation systems, in which glove integrity testing is also outlined specifically.
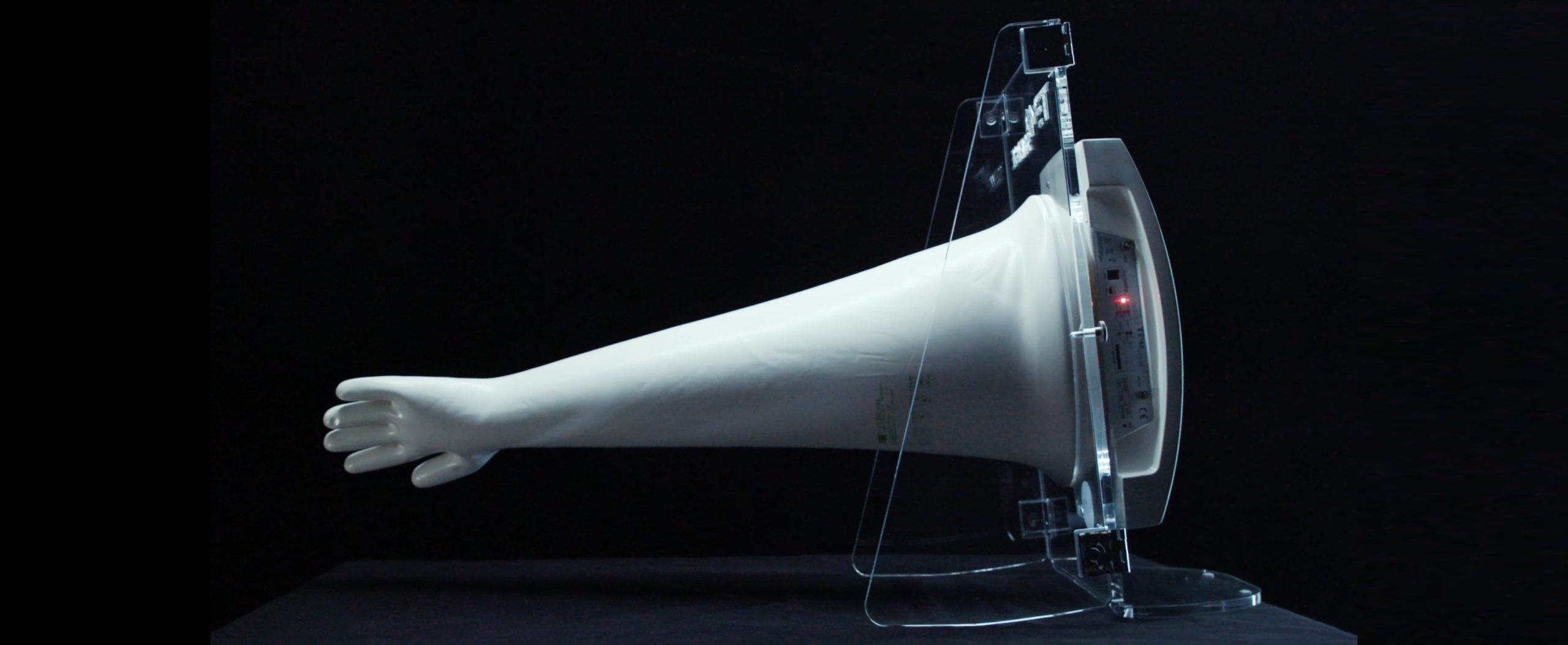
Glove Integrity Testing, increased inspection with Annex 1 revision
The most substantial novelty certainly concerns the definition of the time intervals within which the glove test must be performed, thus defining an aspect that until now was at the discretion of the manufacturer. In fact, the first version of Annex 1, drafted in 2007, states that glove leak tests must take place frequently, but without giving any further specifications.
The newly published revision fills precisely this gap, specifying instead that, as far as isolators are concerned, glove testing must be performed at defined intervals, at least at the beginning and end of each production batch.
“The testing should be performed at defined intervals. Generally, glove integrity testing should be performed at a minimum frequency of the beginning and end of each batch or campaign.1“
In the case of very small hand-processed productions, the criterion is no longer the batch, but rather the manufacturing session.
“For manual aseptic processing activities where single unit or small batch sizes are produced, the frequency of integrity verification may be based on other criteria, such as the beginning and end of each manufacturing session.1”
Clearly, such a change in the regulatory framework cannot fail to have an impact on manufacturing processes. As of 25 August 2023, when the new Annex 1 will come into force, pharmaceutical companies within the European territory, or intending to market their products in this market, will in fact be faced with the need to increase the frequency of glove tests. It is inevitable that in such a scenario, the method used and the technologies adopted to perform the test will play a key role.
Revision of Annex 1 and equipment, some aspects not to be underestimated
As we have seen, the choice of the right glove integrity testing equipment is a key aspect within the manufacturing process. As it ‘demonstrated to be suitable for the task and criticality’, as the revised Annex 1 states, there are also some technical and usage characteristics that should not be underestimated, with reference to the new regulatory framework.
Let’s see some of them.
Reliability: avoiding false positives and false negatives
With the increase in the number of tests depending on production batches, it is even more crucial to avoid the risk of running into both false positives and false negatives as much as possible. In both cases, the result would certainly be the interruption of production until the problem is identified and resolved. In the worst case scenario, it might even be necessary to destroy the entire batch concerned.
In this sense, assessing reliability thoroughly before choosing the equipment could avoid possible future problems, difficult to handle.
The set-up of the equipment also affects the speed of the testing activity
There is no doubt that speed during testing plays a key role in not slowing down the production cycle. But this is not the only parameter to be taken into account: instrument set-up times are also important and can play a key role in streamlining the procedure.
When evaluating the equipment, it is therefore advisable to take this aspect into account as well, and to evaluate not only the actual test time, but also the time required to set up the equipment.
Ease of use: not to be underestimated is also the ease of cleaning and maintenance
A final aspect not to be underestimated is the ergonomics and user-friendliness of the equipment. Certainly a user-friendly instrument will enable the operator to perform the required operations at best in the shortest possible time.
But this is not the only aspect to be taken into consideration. Maintenance is also crucial. Specifically cleaning. Since this is an instrument used almost exclusively in classified environments, it is advisable to evaluate this aspect as well, since an easy-to-clean instrument will take less time to be ready for use, thus saving time and resources.
Discover AGLTS, our automatic Glove Leak Tester
