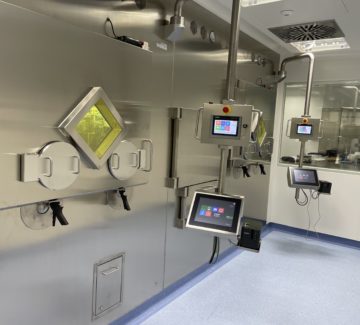
In the SWAN Isotopen’s laboratories, at Bern University Hospital, there is a new radiopharmaceutical production line coming into operation. This line is dedicated both to the development of new formulas requested by medical prescription and to the synthesis-and-dispensing process of radiopharmaceutical based on [18F] e [177Lu].
The new Radiopharmaceutical Production Line realized by Tema Sinergie for SWAN Isotopen, swiss company leader and specialized in the production of radiopharmaceuticals for Nuclear Medicine, has been designed and developed to reach a complete and avantgarde management in the production, handling, and dispensing process of various radiopharmaceuticals, designated both to diagnosis and therapy.
The whole workflow, from the entrance in line of the radioisotopes, to the bio-decontamination cycles, automatic dispensing and data processing, has been meticulously designed and developed in compliance with SWAN’s production needs and the current GMP guidelines.
The result is a maximized and compact Radiopharmaceutical Production Line made by three main segments, that allow to manage the different phases of the production process following specific requests, as underlined by Dr. Konrade von Bremen CEO and Riccardo Bosi Head of Infrastructure & Operation at SWAN Isotopen AG.
“The professionalism of its personnel and the possibility to completely customize the equipment are just some of the strengths, for which we consider Tema Sinergie as our choice. For us it is the best solution for the implementation of new production lines.
The long-standing cooperation between SWAN Isotopen and Tema Sinergie has resulted in an efficient and optimized production process in compliance with current guidelines, ISO and general cGMP regulations.
SWAN Isotopen’s production site is fully equipped with Tema equipment and environmental control systems, which have been developed and produced by Tema Sinergie over the years. After 10 years of continuous production activity, we are happy to equip our latest Production line with TEMA Sinergie’s newest hot cells.
We hope to have the pleasure of working with TEMA Sinergie on other promising future projects.”