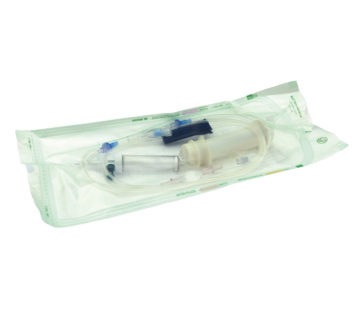
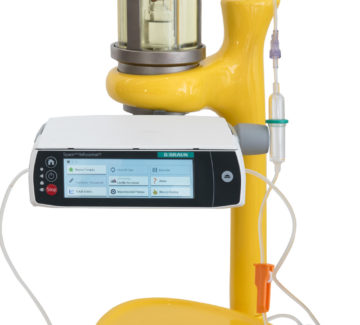
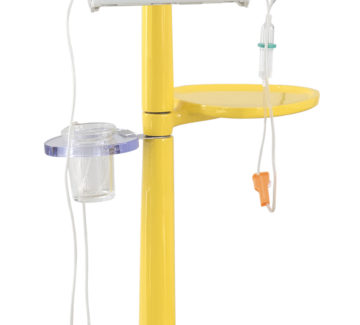
An innovative solution, capable of transforming the management processes of radiopharmaceuticals used in theragnostics and making the administration of radiometabolic therapies simpler and safer.
MEDICAL DEVICE CERTIFICATION PENDING
ASTER is a concept for the infusion of radiopharmaceuticals and will be available on the market starting from 2023.
Fill the form to find out when it will be available in your country: stay tuned
In the last few years, the development of effective radiometabolic therapies has led to the emergence of the Theragnostic approach as a specific application of nuclear medicine.
Very quickly, from being an experimental therapy, Theragnostics is gradually becoming a treatment protocol increasingly used by nuclear medicine departments. And with the increase in treatments, the critical issues associated with a completely manual handling of radiopharmaceuticals and administration to patients could result in complications for the entire department.
ASTER was created to respond to these critical issues and to support those nuclear medicine departments wishing to increase their Theragnostic activity or start new treatments with radiometabolic therapies. Find out how